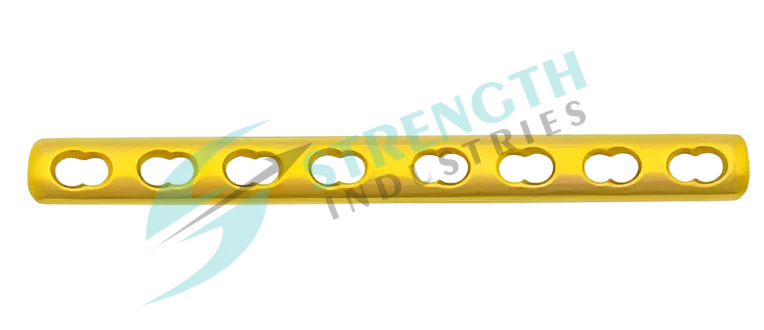
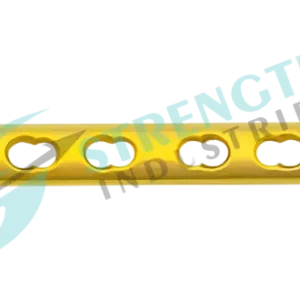
The 1/3 Tubular Locking Plate is a high-performance orthopedic implant designed for internal fixation of small bone fractures. Manufactured using advanced machining technologies, this plate offers precise anatomical contouring, reducing soft tissue interference, and ensuring optimal fit on the bone surface. Compatible with 3.5mm Locking Screws and 3.5mm Cortical Screws, it provides surgeons with versatile fixation options for diverse clinical scenarios.
Product Design & Engineering Features:
- Low-profile anatomical design for minimal soft tissue irritation.
- Combination holes for both locking and compression screw techniques.
- Precision machined screw holes ensuring optimal screw-plate interface.
- Uniform 2mm thickness and 10mm width across all variants.
- Hole configurations available in: 5H (71mm), 6H (84mm), 7H (97mm), 8H (110mm), 9H (123mm), 10H (136mm), 11H (149mm), 12H (162mm).
Material Composition:
- Surgical Grade Stainless Steel (AISI 316LVM).
- Titanium Alloy (Ti-6Al-4V ELI) for superior biocompatibility.
Mechanical Performance:
- High stability under load-sharing and load-bearing conditions.
- Angular stability through locking mechanism for osteoporotic bones.
- Enables stable fracture compression for enhanced bone healing.
Clinical Indications:
- Diaphyseal and metaphyseal fractures of small bones.
- Periarticular fractures with limited soft tissue coverage.
- Corrective osteotomies and deformity corrections.
- Non-unions and complex fracture patterns requiring hybrid fixation.
Compliance & Certifications:
- ISO 13485 Certified Manufacturing Facility.
- CE Marked in compliance with EU MDR.
- Follows AO Fracture Fixation Principles.
Instrument Compatibility:
- Compatible with standard 3.5mm orthopedic screwdrivers and instruments.
- Locking holes designed for standard locking screw heads with hex drive interfaces.














Reviews
There are no reviews yet.